Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a life-threatening disease of dysregulated complement activation. It is a rare disease with an estimated incidence of 1 to 1.5 cases per million people globally. Eculizumab is a humanized monoclonal anti-complement component 5 antibody that was approved for the treatment of patients with PNH in the United States and European Union in 2007, yet unmet medical needs remain. Up to half of patients continue to require blood transfusions despite treatment with eculizumab, and hemolytic activity remains detectable in many patients (Brodsky et al. Blood. 2008). Eculizumab is not available in many countries. In places where treatment is approved, there are further impediments to access, such as cost of treatment, reimbursement issues, infrastructure limitations, and patient restrictions (Risitano et al. Am J Hematol. 2018; Risitano et al. Front Immunol. 2019). Data published on real-world outcomes of eculizumab are limited. Here we describe a study that will retroactively analyze data from patients with PNH treated with eculizumab at the Essen University Hospital in Germany.
Study Design and Methods
This retrospective, secondary data use, cohort study will include all patients at the Essen University Hospital who were diagnosed with PNH and treated with eculizumab prior to April 2018. Clinical data from medical records were entered into an electronic case report form (eCRF). Source data verification has been performed for all clinical data. Laboratory data were extracted directly from the hospital computer system. The Essen University hospital also checked and verified missing laboratory data. Patient-level data in the eCRF and laboratory data were fully anonymized. The primary objective of the study is to understand the remaining unmet medical need by describing the eculizumab dose and frequency of dose adjustment and describing the proportions of patients who experience intravascular and extravascular hemolysis while on treatment. The secondary objectives include explorations of the association between lactate dehydrogenase (LDH) and hemoglobin stabilization with clinical outcomes (eg, breakthrough hemolysis and the need for red blood cell transfusion), the association between PNH clone size and clinical outcomes and the risk of thrombosis, the changes in LDH and hemoglobin levels over time, the need for red blood cell transfusion during eculizumab treatment, and the proportion of eculizumab-treated patients with positive monospecific Coombs test results. In addition, opportunities to apply machine-learning methodologies to predict patients who may not respond to eculizumab will be explored. Many of the analyses will be descriptive. The associations between LDH and hemoglobin with clinical outcomes will be evaluated using rank correlation coefficients or logistic regression. Multivariable regression will be used to explore the prognostic value of clone size on clinical outcomes and thrombosis events.
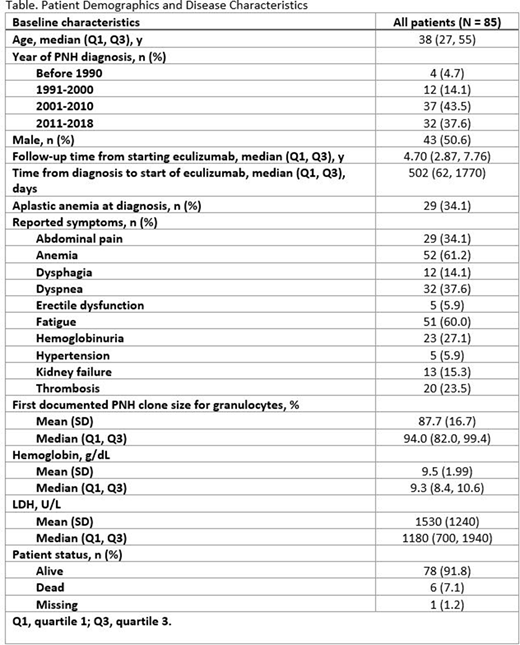
This retrospective study includes 85 patients with PNH with complete clinical and laboratory data (Table). The median age of the cohort was 38 years old, and the cohort was split evenly between men and women. Many patients received a diagnosis of PNH prior to the availability of eculizumab, as the year of diagnosis was 2010 or earlier for 53 patients (62%). The median years of follow-up from initiation of eculizumab was 4.7 years. Overall, 34% had aplastic anemia at diagnosis, and symptoms of fatigue, abdominal pain, and kidney failure were reported in 60%, 34%, and 15%, respectively. At data cutoff, 92% of patients were still alive.
Summary
The patient demographics in this study are comparable to other studies in PNH, suggesting a representative population. With median follow-up time of nearly 5 years, this study will allow for a long-term assessment of the patient experience with eculizumab. High-quality study data is ensured via full source data verification of clinical data and verification of missing laboratory data. These study results will help to address many research questions in PNH, identify the remaining unmet medical need, and also inform new drug development.
Versmold:F. Hoffmann-La Roche Ltd: Other: All authors received support for third party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Raiser:F. Hoffmann-La Roche Ltd: Other: All authors received support for third party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Faghmous:F. Hoffmann-La Roche Ltd: Ended employment in the past 24 months, Other: All authors received medical writing support for this abstract, furnished by Scott Battle, funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. ; Kite Pharma: Current Employment. Katz:F. Hoffmann-La Roche Ltd: Current Employment, Other: All authors received support for third party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Shang:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company, Other: All authors received support for third party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Xu:F. Hoffmann-La Roche Ltd: Current Employment, Other: All authors received support for third party writing assistance, furnished by Scott Battle, PhD, provided by F. Hoffmann-La Roche, Basel, Switzerland.. Röth:Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Biocryst: Consultancy, Honoraria; Apellis: Consultancy, Honoraria; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal